Gait analysis is the study of the manner of walking. It is used worldwide to aid in the diagnosis and treatment of a wide range of ambulatory problems, including Parkinson’s disease, muscular dystrophy, and nerve injury. It is increasing being recognized as a useful preclinical assay to better understand how animal models may reflect the human condition, and to generate quantitative metrics of normal and abnormal gait.
Gait can be assessed in subjects walking voluntarily or on a treadmill. As in human life, however, when subjects walk voluntarily, the assessment of gait can be difficult depending on the subject’s speed.
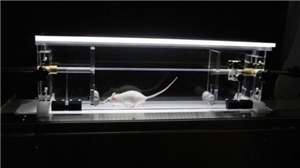
DigiGait is now the most widely published ventral plane videography instrumentation available for gait analysis in laboratory animals. Voluntary and treadmill walking, DigiGait performs gait analysis of mice and rats over a range of walking and running speeds. Ventral Plane Imaging (VPI) Technology continuously images the underside of the animals walking atop of the patented motorized transparent treadmill belt, generating “digital paw prints” and dynamic gait signals. These dynamic gait signals, generated for each of the 4 limbs, describe the posture and kinematics of the animals that reflect strength, balance, and coordination. Animals can also be challenged to walk up an incline or down a decline to maximize the details about the subjects’ motor abilities.
How does DigiGait work?
DigiGait’s patented VPI Technology images the ventral view of animals as they walk on a motorized transparent treadmill belt. [By analogy, imagine yourself at the gym on a treadmill with clear belt, and a camera imaging the underside of your sneakers.] VPI Technology automatically computes the area of the advancing and retreating paws to quantify the spatial and temporal indices of gait. When the motor speed is set to zero, the animals are free to voluntarily traverse the walking corridor, from which images are captured and results generated. The motorized belt, however, enables numerous strides to be captured over a range of walking speeds (0.1 cm/s to 99.9 cm/s); goal boxes and other artifices to compel the animals to walk are not needed. Numerous strides at known and comparable speeds can be measured for all of the animals in a study. Over 50 indices of gait for each limb are reported in spreadsheet ready format.
Why use DigiGait?
DigiGait is the most widely applicable and comprehensive gait analysis instrumentation available. Key advantages over other devices you are considering:
- Enables study of voluntary and treadmill walking
- Enables study of hopping and running at high speeds
- Enables study of gait in animals walking up inclines and down declines
- Enables the capture of numerous strides at known and comparable speeds, eliminating speed differences as the most important confounder in the interpretation of gait differences
- Data generated have lower standard errors and a higher degree of accuracy and repeatability
- The entire ventral view of the subject is imaged, not just the portions of the paw that make contact with illuminated glass
- Higher throughput, for data collection and data analysis
- Early detection of subtle phenotypes; most subtle phenotypes require animals to be studied over numerous strides under challenging conditions, such as is often the case for humans
- Motor Rater
- Fine motor skills assessment
Who else uses DigiGait?
DigiGait is used worldwide at universities, biotechnology companies, and pharmaceutical giants. Clients include Harvard, Yale, Stanford, King’s College London, Biogen, Genzyme, and Pfizer to name a few (please ask for references as we respect our customers’ confidences). Research areas include amyotrophic lateral sclerosis, spinal cord injury, arthritis, traumatic brain injury, and nerve injury…to name a few…
If you are interested in more details or a price quote please Contact Us








Request
Catalogue
Chat
Print