

MOM
Movable Objective Microscope- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview

There are 1 images available to view - click to enlarge and scroll through the product gallery.
MOM DataSheet
/ Download as PDF
Applications
- In vivo two-photon imaging
- Electrophysiological recording and imaging (culture, large in vivo preparations, etc.)
- Non-horizontal surface microscopy
- Simultaneous retinal stimulation and two photon microscopy1
Features
- Objective moves 22mm in X, Y and Z
- Objective rotates about optical axis for imaging of non-horizontal surfaces and volumes
- Customizable open platform design
- Cambridge Technology conventional or resonant XY scanners
- Two or four channel detector system with Hamamatsu PMTs and preamplifiers
- Sutter PS-2/ PS-2LV dual channel PMT power supply
- National Instruments based data acquisition system
The Movable Objective Microscope (MOM) is a two-photon microscope capable of imaging deep within living specimens when combined with a Ti:Sapphire Laser. The MOM design is unique in providing 3-dimensional objective movement and rotation allowing the specimen to remain stationary. Many highly regarded imaging laboratories around the world use MOM microscopes and we constantly work with our customers to adapt the design for their changing needs.
Starting in 2013, we began offering the MOM with resonant scanners. When coupled With the newest version of the MOM Computer System Software (MCS 2.0) it is now possible to image 512 x 512 pixel, full frames at 31 frames per second (FPS). Smaller frames can be scanned at rates higher than 100fps (512 x 32 lines @ 495 fps, 512 x 28 lines @ 566 fps). An advanced streaming system allows continuous saving of more than 150 GB of mixed two-photo imaging and electrophysiology data into a single file on disk. The resonant version of MCS can be easily switched back and forth between conventional and resonant-scanning modes, making resonant scanning an appropriate add on to an existing MOM with conventional scanners. MSC 2.0 with resonant scanning incorporates all the features of the original MCS and is thus designed to take full advantage of the MOM. When combined with the MCS 2.0, The MOM becomes a powerful tool capable of combining electrophysiology with large scale imaging experiments in both two and three dimensions.
How it Works:
The MOM consists of two independent microscopes. The wide-field half of the microscope consists of an Olympus vertical illuminator, Sutter Xenon arc lamp and camera mount to provide standard epifluorescence. The two-photon side of the microscope provides the optical pathway for guiding the excitation laser light from the table up into the scanning galvanometric mirrors and then expanding the beam through the scan lens and directing into the back of the objective. Following two-photon excitation, the emitted photons are directed by a dichroic mirror immediately above the objective into the detection pathway. The main body of the microscope translates on a rail system to allow easy access to the specimen prior to imaging.
The objective translates in X, Y and Z as well as rotates around the X axis. Two moving mirrors allow the microscope to maintain efficient delivery of the excitation light to the back aperture of the objective regardless of movement or orientation. The X, Y and Z movements used are the same as that in our MP-285 micromanipulator so you know the movements are smooth, fine in scale, drift-free and highly reproducible. These movements permit Z-stacks and mosaic images of large regions of tissue to be recorded wihtout the need for a moving stage.
The horizontal light path allows for rotation of the objective away from the standard vertical position. As a result of this rotation, the MOM can easily be converted from an upright to an inverted microscope and the objective positioned from 0 to 180 degrees. This positional freedom permits the imaging of non-horizontal surfaces and volumes.
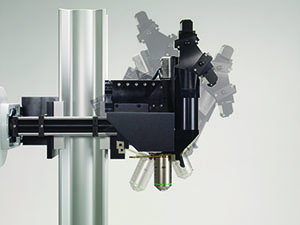
Sutter MOM packages include all of the equipment (less the Ti:Sapphire laser and objective) needed for a complete imaging system.
Cambridge Technology XY galvanometric scanners
(conventional with 3 or 6 mm mirrors or resonant with 5mm mirrors)
Hamamatsu photomultiplier tubes (PMTs):
R6357 multialkali or H10770PA-40 (GaAsP) products
(Sutter is an authorized reseller for Hamamatsu)
Power supplies for PMTs:
Either a Sutter PS-2 (dual channel high-voltage power supply for R6357 PMTs) or
Sutter PS-2LV (dual channel low-voltage power supply for H10770PA-40 (GaAsP) PMTs) can be ordered
Hamamatsu C7319 or Sigmann pre-amplifiers
Data acquisition: National Instruments acquisition boards (NI-6110E)
VIDEOS
MOM scope translation to allow easy access to preparation
Rotation of MOM head to image non-horizontal surfaces
MOM Objective movement in X, Y and Z
1 "Eyecup scope-optical recordings of light stimulus-evoked flourescence signals in the retina", Euler et al, Pflugers Arch, 2008
Specifications
TECHNICAL SPECIFICATIONS
Dimensions
30 in x 14.25 in x 13.25 in | 76 cm x 36 cm x 33.5 cm
Weight
80 lbs | 36 kg
Electrical
115/230 volts
50/60 Hertz power line
RoHS Compliant
MOM - BASIC SYSTEM FOR 2-PHOTON MICROSCOPY
Includes Moving Objective Microscope, 2 channel detector with PMTs, preamps and PS-2 power supply, XY scanners with servo drive, wide field fluorescence unit including vertical illuminator, LB-LS 300 Watt Xenon Arc Lamp, LLG and light guide adapter, C-mount for wide field camera, data acquisition system.
Catalog Number and Description
MOM-3mm1
MOM System with 3mm XY scanners
MOM-6mm1
MOM System with 6mm XY scanners
MOM-RESONANT1
MOM System with Resonant scanners and MCS 2.0
1 Final pricing depends on detector path selected and does not include several devices necessary for a complete 2-photon microscope (i.e. Ti:Sapphire laser, objective, camera, trinocular head, table mount optics). Please phone WPI for details.
Accessories
Citations
Castilho, Á., & Ambrósio, A. (2015). Disruption of a Neural Microcircuit in the Rod Pathway of the Mammalian Retina by Diabetes Mellitus. The Journal of …. Retrieved from https://www.jneurosci.org/content/35/13/5422.short
Coiro, P., Padmashri, R., & Suresh, A. (2015). Impaired Synaptic Development in a Maternal Immune Activation Mouse Model of Neurodevelopmental Disorders. Brain, Behavior, and …. Retrieved from https://www.sciencedirect.com/science/article/pii/S0889159115004171
Reiner, B., & Dunaevsky, A. (2015). Deficit in Motor Training-Induced Clustering, but Not Stabilization, of New Dendritic Spines in fmr1 Knock-Out Mice. Retrieved from https://dx.plos.org/10.1371/journal.pone.0126572
Anwar, H., & Roome, C. (2014). Dendritic diameters affect the spatial variability of intracellular calcium dynamics in computer models. Name: Frontiers in …. Retrieved from https://journal.frontiersin.org/Journal/10.3389/fncel.2014.00168/pdf
Eggermann, E., Kremer, Y., Crochet, S., & Petersen, C. (2014). Cholinergic Signals in Mouse Barrel Cortex during Active Whisker Sensing. Cell Reports. Retrieved from https://www.sciencedirect.com/science/article/pii/S2211124714009590
Letourneur, A., & Chen, V. (2014). A method for longitudinal, transcranial imaging of blood flow and remodeling of the cerebral vasculature in postnatal mice. Physiological …. Retrieved from https://physreports.physiology.org/content/2/12/e12238.abstract
Park, J., Frantz, M., Kast, R., & Chapman, K. (2014). Nogo Receptor 1 Limits Tactile Task Performance Independent of Basal Anatomical Plasticity. PloS One. Retrieved from https://dx.plos.org/10.1371/journal.pone.0112678
Paukert, M., Agarwal, A., Cha, J., & Doze, V. (2014). Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. Retrieved from https://www.sciencedirect.com/science/article/pii/S0896627314003535
Roome, C., & Kuhn, B. (2014). Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Frontiers in Cellular Neuroscience. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4227473/
Seemann, K., & Kuhn, B. (2014). Multi-photon excited luminescence of magnetic FePt core-shell nanoparticles. Biomedical Optics Express. Retrieved from https://www.opticsinfobase.org/boe/fulltext.cfm?uri=boe-5-7-2446&id=294401
Suzuki, N., Tang, C., & Bekkers, J. (2014). Persistent barrage firing in cortical interneurons can be induced in vivo and may be important for the suppression of epileptiform activity. Frontiers in Cellular …. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3952511/
Tada, M., & Takeuchi, A. (2014). A highly sensitive fluorescent indicator dye for calcium imaging of neural activity in vitro and in vivo. European Journal of …. Retrieved from https://onlinelibrary.wiley.com/doi/10.1111/ejn.12476/full





Request
Catalogue
Chat
Print