
TEER for Organ-on-Chip Platforms
Transepithelial Electrical Resistance (TEER) measurements play a vital role in assessing barrier function, tissue integrity, and cell-cell interactions in Organ-on-Chip (OoC) platforms. Measuring TEER by applying a small AC current across a monolayer of cells and measuring the resistance to that current, which provides valuable insights into the physiological relevance and functionality of in vitro models, enabling researchers to study organ-specific responses, disease mechanisms, and drug effects while cells are growing in a controlled microenvironment. This article will explore the applications of TEER in OoC set-ups, critical aspects to TEER measurements in microphysiological systems, and challenges and limitations that must be overcome to fully realize the potential of this technology for OoC platforms.
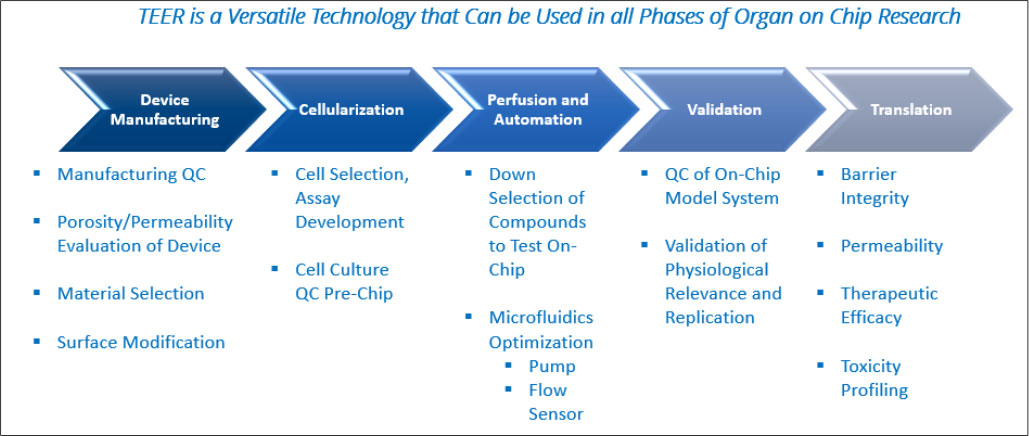
Applications of TEER in OoC Platforms
Manufacturing QC
TEER measurements are often utilized to establish the baseline parameters of an OoC system prior to an experiment. By regularly monitoring TEER values at various stages of production, manufacturers can assess the consistency and reproducibility of the cell barrier properties across multiple OoC devices. TEER can measure the porosity of a membrane within an OoC setup and can also establish when seeded cells have formed a mature barrier and are ready for testing. As OoC applications are scaled up and standardized, the importance of implementing QC into the manufacturing process becomes even more critical and TEER is a facile and cost-effective way to assure the OoC systems are manufactured correctly and meet specifications.
Barrier Function Assessment
TEER measurement is widely used to evaluate the barrier properties of epithelial and endothelial cell layers in OoC models. By monitoring changes in TEER values over time, researchers can assess the tightness of cell junctions, permeability of barriers, and integrity of tissue structures. TEER measurements are essential for studying drug transport, immune responses, and disease progression in organ-specific OoC platforms.
Drug Permeability Studies
TEER measurement is instrumental in studying drug permeability across epithelial and endothelial barriers, such as the blood-brain barrier, gut epithelium, and lung alveolar epithelium. Changes in TEER values can indicate alterations in barrier function, drug transport mechanisms, and cellular responses to pharmacological agents. TEER measurements provide quantitative data on drug absorption, distribution, metabolism, and excretion in OoC models, enabling drug screening and toxicity testing.
Disease Modeling and Pathophysiology
TEER measurement enables researchers to monitor changes to barrier tissues associated with diseased conditions, pathological changes, and inflammatory responses in OoC models. By monitoring TEER values in real-time, researchers can simulate disease-specific conditions, such as inflammation, infection, and tissue damage, to study disease mechanisms and therapeutic interventions. TEER measurements offer insights into disease progression, tissue remodeling, and drug efficacy in organ-specific OoC platforms.
Critical Aspects of TEER Measurements in OoC Platforms
Electrode Configuration
TEER measurements in OoC platforms are typically performed using chopstick electrode configurations that apply a small electrical current across the cell layer and measure the resulting resistance. These chopstick electrodes are handheld devices, inserted into the OoC to measure TEER at any given time. These TEER measurement setups work well for systems, where timepoints are spaced widely apart (days-weeks) and can enable higher throughput readings, albeit manual. While every OoC is designed differently, only a few parameters need to be optimized for chopstick electrodes to work for TEER measurement. These are the distance between electrodes and the depth that the electrodes must go into the chip. Once those parameters are determined, customized chopsticks can be made to measure TEER on many OoC systems. Because these are re-usable electrodes, the cost is much less for this solution.
Real-Time Monitoring
TEER measurements can be conducted in real-time using impedance spectroscopy and measurement techniques that rely on embedded electrodes within the OoC system. Real-time monitoring of TEER values enables researchers to assess dynamic changes in barrier function, cellular responses, and drug effects in OoC platforms. Continuous TEER measurements provide valuable temporal data on cellular behavior, tissue integrity, and drug interactions in organ-specific models. In systems where TEER is measured continuously and in real time, embedded electrode systems must be highly customized depending on the geometry of the OoC system, where the microfluidic layers reside, and how the cell chambers are arranged. Materials for these embedded electrodes are often made of gold or platinum and enable long-term and continuous reading on chips. Parameters such as electrode material, electrode size and geometry, and techniques to deposit metal electrodes onto glass all must be optimized for a given OoC.
Challenges and Limitations of TEER Technology in OoC Platforms
Customization
Each OoC system is designed uniquely, making the type of TEER (chopstick versus embedded) unique to each system, and the design of the electrodes must also be customized to provide the best TEER measurements in any given system. While many of the key parameters have been established, each OoC needs to be tested and validated to ensure that TEER measurements are reproducible and reliable.
Variability and Sensitivity
TEER measurements in OoC platforms may exhibit variability and sensitivity to experimental conditions, cell types, and culture environments. Factors such as temperature fluctuations, media composition, and electrode positioning can affect TEER values and introduce measurement errors in OoC studies. Standardization of TEER measurement protocols and quality control measures is essential to minimize variability and ensure reproducibility in OoC platforms.
Single-Cell Analysis
TEER measurements in OoC models provide bulk resistance values across cell layers, limiting the ability to perform single-cell analysis and assess heterogeneity within cell populations. Obtaining spatially resolved TEER measurements and characterizing individual cell responses in OoC platforms can enhance the understanding of cellular interactions, barrier properties, and drug responses in complex tissue models.
Long-Term Stability
TEER measurements in OoC platforms may exhibit changes in resistance values over extended culture periods, affecting the reliability and consistency of data obtained from long-term studies. Maintaining cell viability, barrier function, and tissue integrity in OoC models is critical for ensuring the long-term stability of TEER measurements and the relevance of experimental outcomes in organ-specific platforms.
In conclusion, TEER measurement serves as a valuable tool for assessing barrier function, drug permeability, and tissue integrity in OoC platforms. By monitoring TEER values in real-time, researchers can study organ-specific responses, disease mechanisms, and drug effects in a controlled microenvironment. Despite its applications and benefits, TEER technology in OoC platforms faces limitations related to variability, sensitivity, single-cell analysis, and long-term stability, all of which are being actively addressed. Addressing these challenges and advancing TEER measurement techniques in OoC research can enhance the reliability, reproducibility, and predictive value of in vitro models for drug development, disease modeling, and personalized medicine applications.
References
1. Kim, H. et al. (2020). "Evaluation of barrier function in lung-on-chip platform using transepithelial electrical resistance measurement." Lab on a Chip, 20(9), 1565-1576.
2. Zhang, B. et al. (2019). "Transepithelial electrical resistance measurement in organ-on-chip platforms for drug permeability studies." Advanced Drug Delivery Reviews, 149, 65-80.
3. Maschmeyer, I. et al. (2015). "A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents." Lab on a Chip, 15(12), 2688-2699.




Request
Catalogue
Chat
Print