Genetic Transfer (viral or non-viral vector) Using Microprocessor-Controlled Injector
With the first approved human gene therapy trial in 1989(1) (Rosenberg, et.al.), gene therapy has come a long way in modern medicine and is making inroads in clinics and the market in general (2), (3). 2017 was an important year for gene therapy when Luxurna, the first human gene therapy drug for an inherited retinal dystrophy, was approved by Food and Drug Administration (USA) (4). Now several drugs are undergoing clinical trials. With an estimated $11 billion (USD) market in the next 10 years, both clinical trials and the pharmaceutical industry are expected to benefit immensely from gene therapy (5).
With improved use of viral vectors (Adeno-associated virus (AAV), adenovirus, lentivirus, retrovirus, HSV) or non-viral vectors, research and clinical trials on the development of therapeutic genes have witnessed great success. Viral vectors have been employed for the treatment of various diseases such as metabolic, cardiovascular, muscular, hematologic, ophthalmologic and infectious diseases and various types of cancer (6).
Virus induced gene silencing (VIGS) has also found widespread use in deciphering the functions of plant genes involved in biotic and abiotic stress resistance, metabolic and regulatory pathways and reproduction and flowering (7).
For the success of these achievements an injector that can ensure reproducible and efficient delivery of the viral or non-vectors is crucial. WPI’s versatile UltraMicroPump (UMP3) with SMARTouch™ microprocessor-controlled injector and sub-microliter (NanofilTM) injection system that ensures reproducible and precise delivery of microliter to nanoliter volumes with high efficiency have been widely used for transferring viral vectors and have become the standard practice (8), (9), (10)
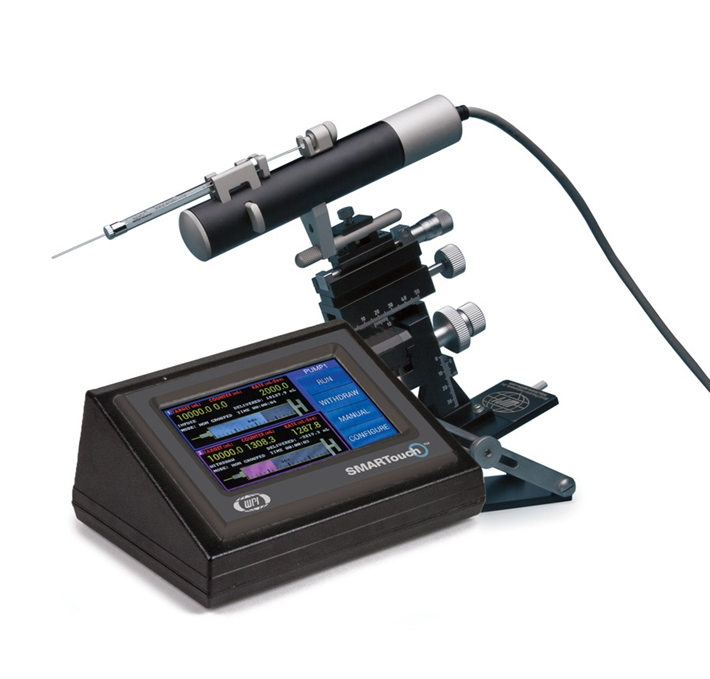
UMP3 Features
- Accepts a wide variety of syringes from 0.5µL to 1.0 mL
- Very low dead volume (0.5 µL or less)
- Graphic display with SMARTouch™ touch screen controller
- Footswitch (optional)
UMP3 Benefits
- Highly versatile and capable of transferring fluids from nanoliter to microliter volumes
- Configure volume and flow rates
- Eliminates waste and makes the system highly efficient
- Rapid set up and "intelligent," easy-to-use interface for controlling up to two UMP3 syringe pumps
- Using the optional footswitch leaves your hands free
References
- Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans – immunotherapy of patients with advancedmelanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990; 323:570–578. DOI:10.1056/NEJM199008303230904
- Corrigan-Curay, J., O’reilly, M., Kohn, D. B., Cannon, P. M., Bao, G., Bushman, F. D., et al. (2015). Genome editing technologies: defining a path to clinic: genomic editing: establishing preclinical toxicology standards, Bethesda, Maryland 10 June 2014. Mol. Ther. 23, 796–806. doi: 10.1038/mt.2015.54
- Friedmann, T. (2007). A decade of accomplishments: gene therapy and the ASGT. Mol. Ther. 15, 1576–1578. doi: 10.1038/sj.mt.6300284
- Dias et al., 2017 Dias, M. F., Joo, K., Kemp, J. A., Fialho, S. L., Da Silva Cunha, A., Woo, S. J., et al. (2018). 22Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 63, 107–131. doi: 10.1016/j.preteyeres.2017.10.004
- Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D,Chawla K, Chattopadhyay S,Chandra D, Chilukuri N and Betapudi V (2019) Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 9:297. doi: 10.3389/fonc.2019.00297
- Kenneth Lundstrom. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42; doi:10.3390/diseases6020042
- Kant, R., Dasgupta, I. Gene silencing approaches through virus-based vectors: speeding up functional genomics in monocots. Plant Mol Biol 100, 3–18 (2019). https://doi.org/10.1007/s11103-019-00854-6
- Andrew I Brooks et. al. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J Neurosci Methods. 1998 Apr 30;80(2):137-47.
- Lowery, R. L., Majewska, A. K. Intracranial Injection of Adeno-associated Viral Vectors. J. Vis. Exp. (45), e2140, doi:10.3791/2140 (2010).
- Inquimbert, P., Moll, M., Kohno, T., Scholz, J. Stereotaxic Injection of a Viral Vector for Conditional Gene Manipulation in the Mouse Spinal Cord. J. Vis. Exp. (73), e50313, doi:10.3791/50313 (2013).




Request
Catalogue
Chat
Print